From Frog Legs to Batteries: How Does an Energy Storage System Work?
A Bit of History: The Discovery of Electricity
Luigi Galvani was an Italian physician and curious scientist in the 18th century. During an experiment investigating frog leg muscles, he discovered a connection between layered metals and the twitching of frog legs. When Galvani accidentally touched the muscles with a brass rod against a metal grid, they twitched. He understood that electricity played a role here – but what exactly happened was only established some years later.
The Italian physicist Alessandro Volta recognised, based on Galvani's discovery, that electrical voltage can be generated using two different metal discs and a leather disc soaked in salt water between them. Volta stacked first a zinc disc, then a damp leather disc, and then a silver disc on top of each other. He repeated this several times and created the Voltaic pile – the first technically usable battery.
In honour of these scientific discoveries:
- The physical unit of voltage (Volt) was named after Volta
- The combination of zinc, silver and leather discs was called the Galvanic cell
The galvanic cell forms the basis for all modern battery cells.
Some Fundamentals: Noble and Base Metals
What is the significance of the combination of silver, zinc and salt-soaked leather? To answer this, some basic terms must first be explained.
The ability of metals to release and absorb electrons is the foundation for understanding how a battery works:
| Metal Type | Properties | Examples |
|---|---|---|
| Noble metals | Do not rust, react little with other substances, retain electrons and can absorb more | Silver, Gold, Platinum |
| Base metals | Very reactive, rust, release electrons | Zinc, Iron, Aluminium |
The Most Important Terms in Battery Technology
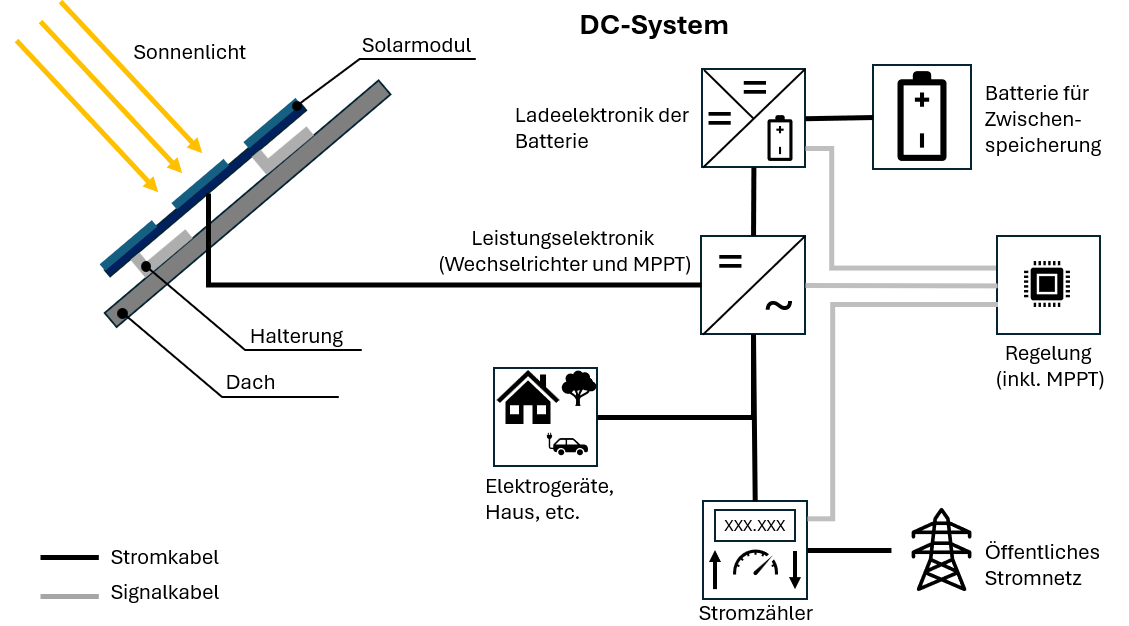
Anode: The negative electrode (minus pole), made of base material, releases electrons.
Cathode: The positive electrode (plus pole), made of noble material, absorbs electrons.
Electrolyte: A liquid or solid that can conduct electrical current with the help of charged particles (ions).
Separator: Separates anode and cathode but allows ions through – prevents short circuit.
Operating Principle in Detail: The Discharge Process
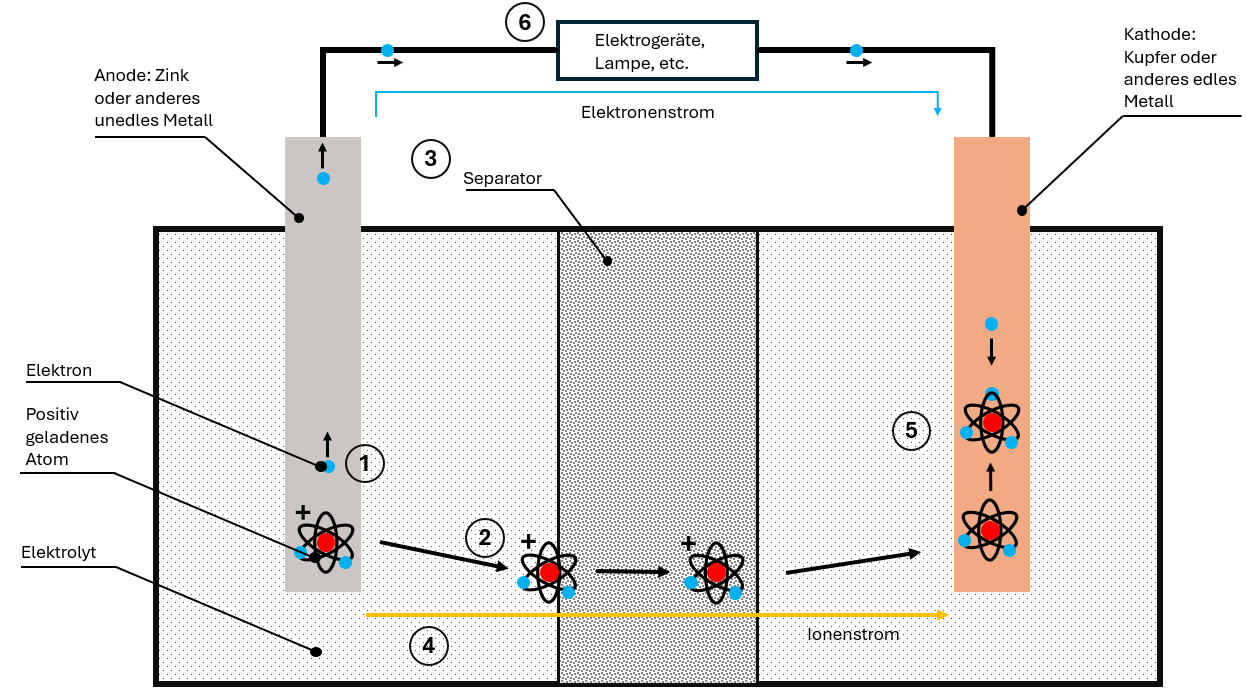
The standard battery cell comprises four components:
- Anode (minus pole)
- Cathode (plus pole)
- Electrolyte
- Separator
How Discharge Works:
- Electron release: The base anode releases electrons that travel through the cable to the cathode
- Ion formation: Due to the electron deficiency, positively charged ions form in the anode
- Ion migration: The ions dissolve in the electrolyte and migrate through the separator to the cathode
- Two separate currents:
- Electron current through the cable (usable!)
- Ion current through the electrolyte
- Reunification: In the cathode, electrons and ions are reunited
The electron current is direct current – it always flows in one direction and can be used for electrical devices. Chemical energy is thus converted into electrical energy.
The Charging Process: Everything in Reverse
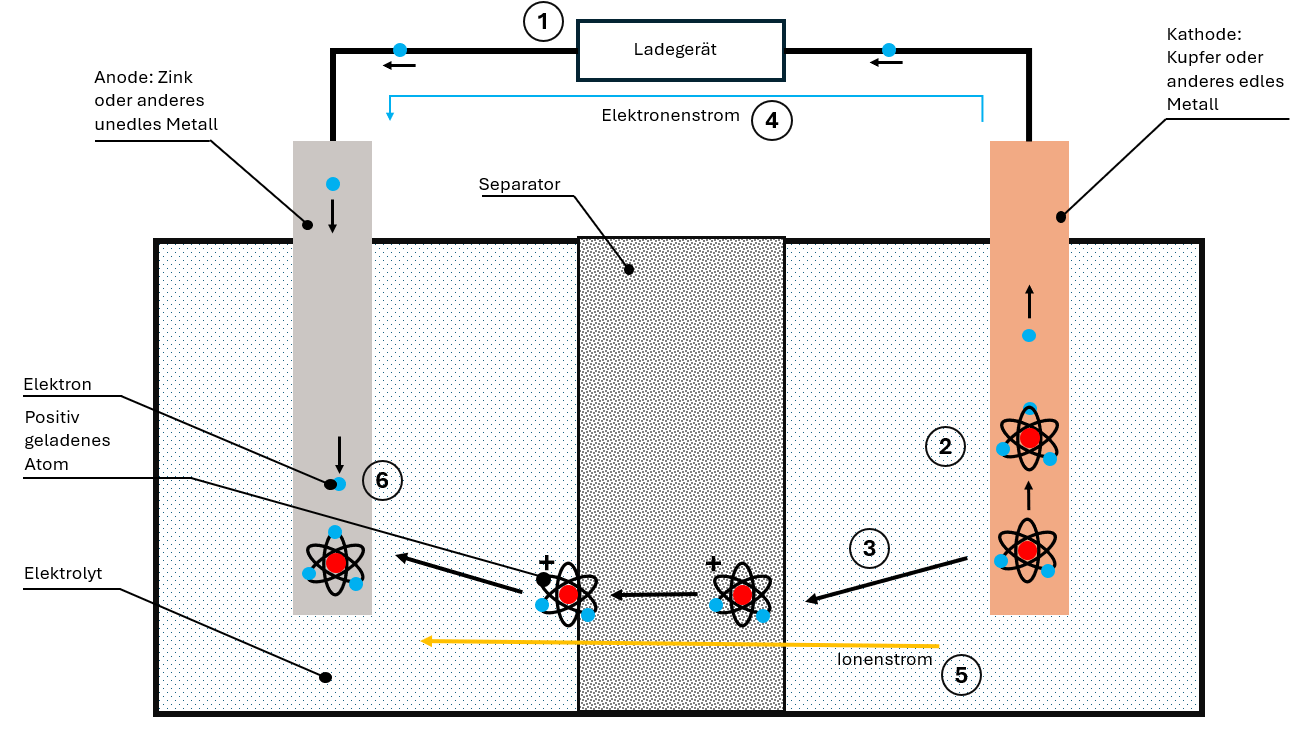
During charging, the charger applies a reverse voltage. This reverses the entire process:
- The cathode "loses" its electrons, which migrate to the anode
- Ions detach from the cathode material
- The ions move through electrolyte and separator to the anode
- In the anode, ions and electrons are stored again
The battery is recharged and ready for the next discharge cycle.
Important Technical Terms and Units
Lifespan and Charge Cycles
A charge cycle is the process of complete discharge and recharge. Modern lithium-ion batteries achieve between 1,000 and 4,000 cycles depending on type.
Depth of Discharge (DoD)
The depth of discharge indicates what percentage of the battery has been discharged:
- 0% DoD = Battery fully charged
- 100% DoD = Battery fully discharged (avoid!)
Each battery type has a maximum recommended depth of discharge. Going below this damages the battery.
C-Rate: Charge and Discharge Speed
The C-rate describes the ratio between charge/discharge power and capacity:
C-rate = Power (kW) / Capacity (kWh)
| C-Rate | Charge/Discharge Time | Meaning |
|---|---|---|
| 0.25C | 4 hours | Gentle charging |
| 0.5C | 2 hours | Standard home storage |
| 1C | 1 hour | Fast charging |
| 2C | 30 minutes | High performance |
Example: A battery with 10 kW power and 20 kWh capacity has a C-rate of 0.5C – it charges or discharges in 2 hours.
Energy Density
The ratio between stored energy and volume:
- Volumetric: Wh per litre (Wh/l)
- Gravimetric: Wh per kilogram (Wh/kg)
Lithium-ion batteries with LFP cathodes achieve approximately 200 Wh/kg – significantly more than lead-acid batteries.
Memory Effect
This phenomenon describes the reduction in capacity from charging a battery that is not fully discharged. The battery "remembers" the shortened charge cycle and only delivers a corresponding portion of its capacity.
Good to know: Modern lithium-ion batteries are practically unaffected by the memory effect – unlike older nickel-cadmium batteries.
Overview: The Most Important Units
For working with batteries and solar systems, some units and metrics are particularly important. The following table summarises the key quantities:
| Unit | Name | Meaning |
|---|---|---|
| kW | Kilowatt | Power (work per time) |
| kWh | Kilowatt-hour | Energy quantity (1 kW for 1 hour) |
| kWp | Kilowatt-peak | Maximum solar system output |
| % (η) | Efficiency | Usable / applied energy |
| C | C-rate | Charge/discharge power / capacity |
| % DoD | Depth of Discharge | Discharge depth |
Conclusion
The Essentials: The operating principle of the battery is based on the galvanic cell and chemical reactions. With an anode, cathode, separator and electrolyte, electrons and ions are separated from each other – creating usable electrical voltage. What began over 200 years ago with frog legs and metal discs is today the foundation for smartphones, electric vehicles and solar systems.
Continue reading: In the next article Lithium vs. Lead: Which Battery for the Solar System?, we compare the two most important battery technologies for use in solar systems.
The Complete Article Series "Energy Storage for Solar Systems"
- From Frog Legs to Batteries: How Does an Energy Storage System Work? – You are here
- Lithium vs. Lead: Which Battery for the Solar System? – Technology comparison
- Power Electronics: Inverters and DC-DC Converters – Power conversion
- The All-Rounder: Hybrid Inverters – Everything in one device
- AC or DC? System Topologies for Solar Systems – System concepts
